Does Alcohol Have Higher Boiling Point Than Phenol Alcohols feature the hydroxyl group attached to a saturated carbon atom in an alkyl chain while phenols have the hydroxyl group directly bonded to an aromatic benzene ring
Alcohols are substantially less volatile have higher melting points and greater water solubility than the corresponding hydrocarbons see Table 15 1 although the differences become Also like water alcohols and phenols have higher boiling points than might be expected because of hydrogen bonding Section 2 12
Does Alcohol Have Higher Boiling Point Than Phenol

Does Alcohol Have Higher Boiling Point Than Phenol
https://i.ytimg.com/vi/XZyezwSHhTQ/maxresdefault.jpg

Which Compound Has A Higher Boiling Point Intermolecular Force Boiling
https://i.ytimg.com/vi/B0gWQQVI9v8/maxresdefault.jpg

HF Has More Boiling Point Than HCl Give Reason p block Elements ASN
https://i.ytimg.com/vi/17o2rapHPLk/maxresdefault.jpg
Phenols are similar to alcohols but form stronger hydrogen bonds Thus they are more soluble in water than alcohols and have higher boiling points Phenols occur either as colourless liquids or white solids at room temperature and may be Phenol has stronger intermolecular forces hydrogen bonding between its molecules compared to alcohol This stronger bonding requires more energy to break apart
Explain why the boiling points of alcohols and phenols are much higher than those of alkanes ethers etc of similar molecular mass discuss the factors that are believed to determine the acidity of alcohols and phenols See how the primary alcohols 1 butanol and 2 methyl 1 propanol have higher boiling points than the secondary alcohol 2 butanol which has a higher boiling point than the tertiary alcohol t butanol
More picture related to Does Alcohol Have Higher Boiling Point Than Phenol

Why Primary Amines Have Higher Boiling Point Than Those Of Tertiary
https://i.ytimg.com/vi/YwSKWX_1gbg/maxresdefault.jpg

JeyleenOconnell
https://o.quizlet.com/HdJQIPAy0-PcXUZAy.8JrA.jpg
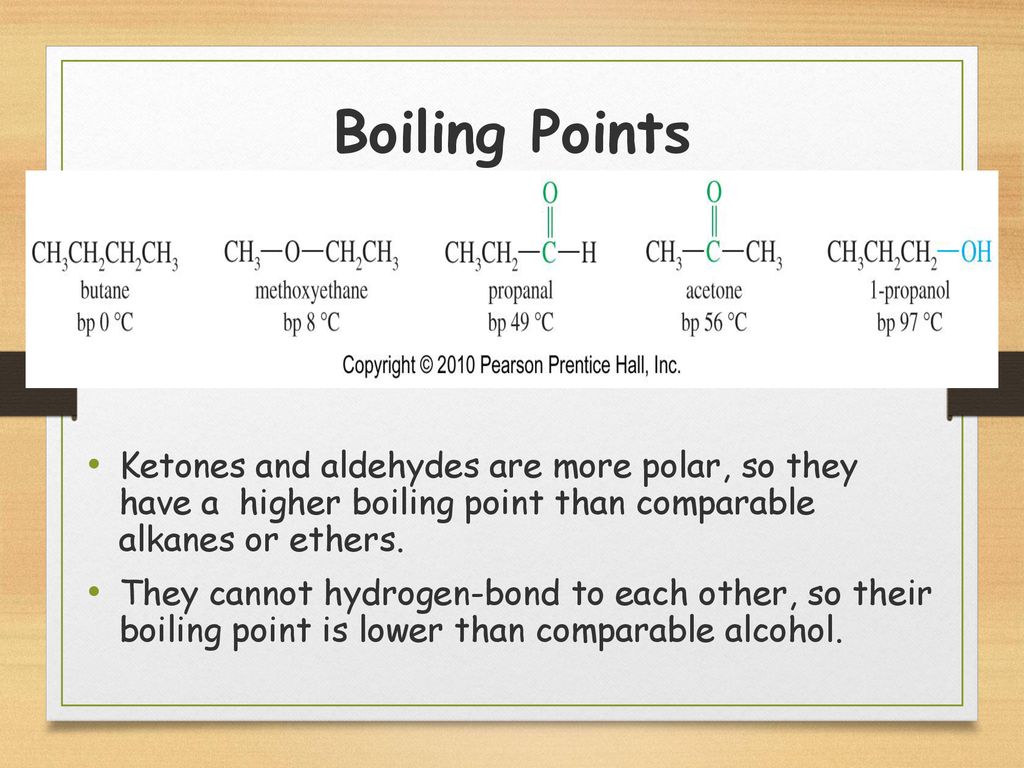
Ketones Aldehydes Amines Ppt Download
https://slideplayer.com/slide/14836546/90/images/11/Boiling+Points+Ketones+and+aldehydes+are+more+polar%2C+so+they+have+a+higher+boiling+point+than+comparable+alkanes+or+ethers..jpg
Also like water alcohols and phenols have higher boiling points than might be expected because of hydrogen bonding Section 2 12 A positively polarized OH hydrogen atom from one molecule is attracted to a Alcohols have higher boiling points than do ethers and alkanes of similar molar masses because the OH group allows alcohol molecules to engage in hydrogen bonding
Therefore they have higher intermolecular interactions and as a result higher boiling points The high values of the boiling points of alcohols compared with alkanes of similar molecular Alcohols boil at higher temperature than alkanes of similar MW The higher boiling point is due to hydrogen bonding If hydrogen is joined to any of the three most electronegative elements

Reason For Carbonic Acid Have Higher Boiling Point Than Alcohol
https://hi-static.z-dn.net/files/d91/02b94837a7ae91586ed4e0223edc1228.jpg

Question Video Identifying The Organic Molecule With The Highest
https://media.nagwa.com/959168746282/en/thumbnail_l.jpeg

https://www.geeksforgeeks.org › difference-between-alcohol-and-phenol
Alcohols feature the hydroxyl group attached to a saturated carbon atom in an alkyl chain while phenols have the hydroxyl group directly bonded to an aromatic benzene ring

https://chem.libretexts.org › Courses › Winona_State...
Alcohols are substantially less volatile have higher melting points and greater water solubility than the corresponding hydrocarbons see Table 15 1 although the differences become

Question Video Understanding The Relationship Between Alkene Chain

Reason For Carbonic Acid Have Higher Boiling Point Than Alcohol
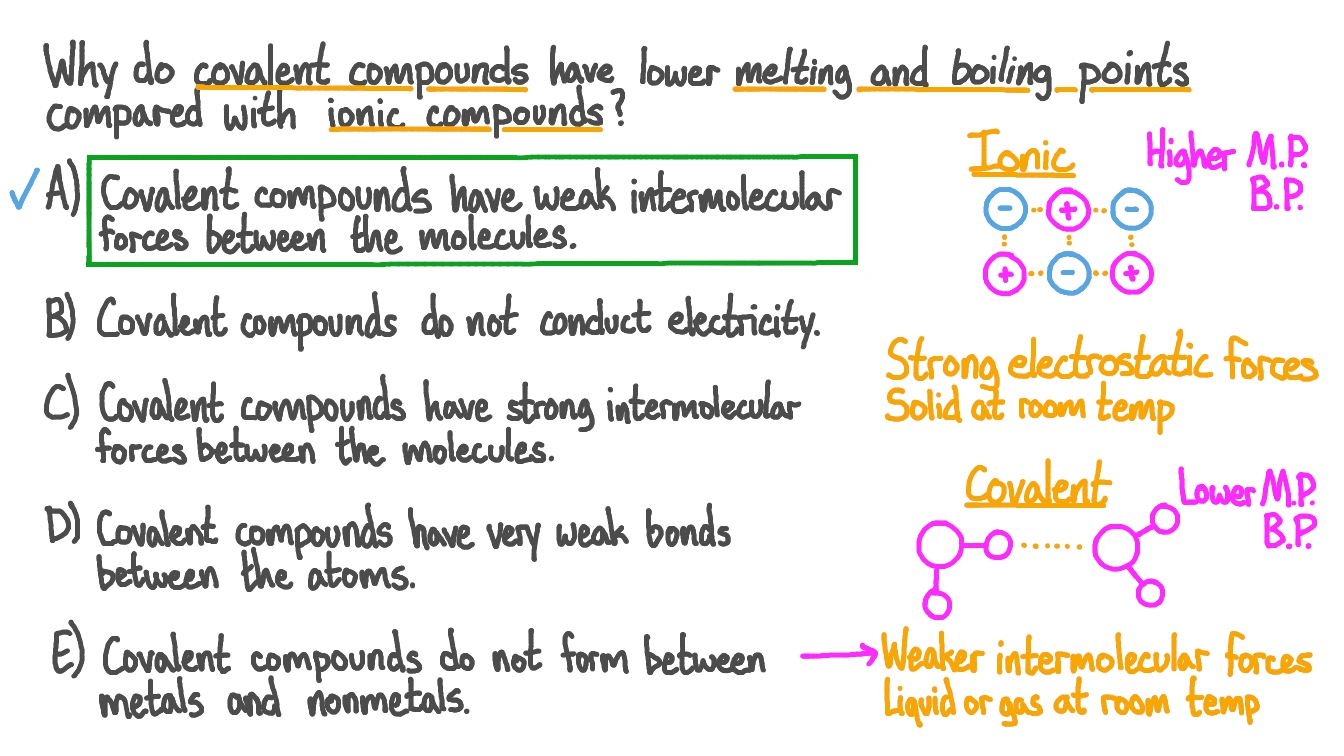
Question Video Determining Why Covalent Compounds Have Low Melting And
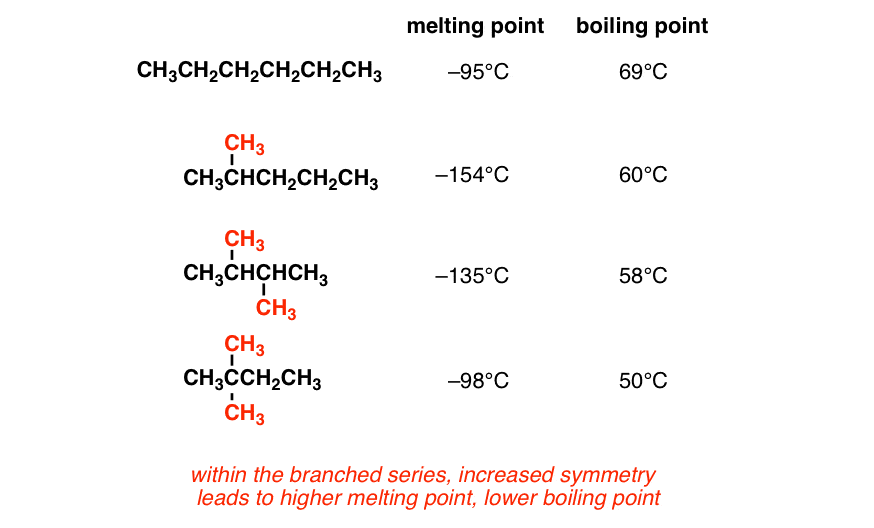
Lead Periodic Table Melting Point Cabinets Matttroy

Pin On CHEMISTRY IS A MYSTERY
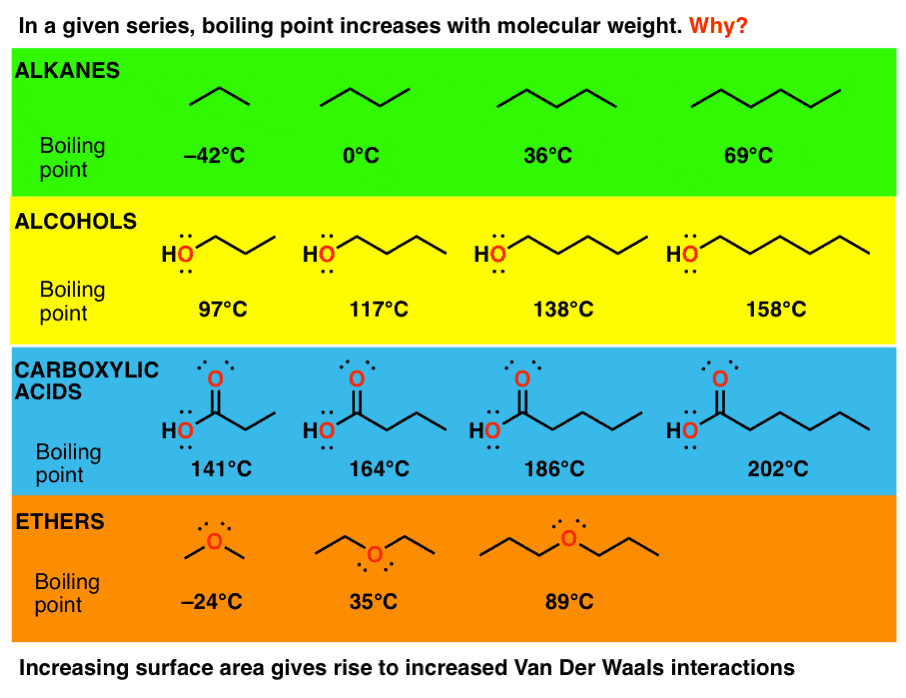
Boiling Point Of Ester And Carboxylic Acid LukafinSwanson

Boiling Point Of Ester And Carboxylic Acid LukafinSwanson

Boiling Point Of Ester And Carboxylic Acid LukafinSwanson

Boiling Point Chemistry

Boiling Point Chemistry
Does Alcohol Have Higher Boiling Point Than Phenol - Phenol has stronger intermolecular forces hydrogen bonding between its molecules compared to alcohol This stronger bonding requires more energy to break apart